Product Literature Standard (PLS) for veterinary medicines
Guidance for the veterinary pharmaceutical industry on the production of mock-ups for assessment.
Introduction
The Product Literature Standard (PLS) should be taken into account when creating mock-ups for assessment.
Taking note of the following will greatly enhance your chances of getting your mock-ups right first time:
- Follow the approved QRD text (if available) or the approved SPC (if no QRD) and information in this document
- If you wish to deviate from the above, contact the relevant authority before submitting mock-ups
- Deviating from the above will only be permitted in very exceptional circumstances – it is up to you to agree the wording during the assessment phase of the application procedure and / or before the mock-up review process
- Minor deviations are permitted to correct grammatical errors
- If you are asked to make changes, please make them and do not make any other changes without agreeing it with the relevant authority first
Definitions
| Type | Details to appear |
|---|---|
| Immediate Packaging | The label on the container or any other form of packaging that is in direct contact with the veterinary medicine. The immediate packaging does not include capsules which are administered as part of the product. |
| Outer Carton/Packaging | The packaging into which the immediate packaging is placed. |
| Label | Information on the immediate or outer packaging. |
| Package Leaflet | The leaflet that contains information for the user which accompanies the product. |
| Product Literature | Consists of labelling for the immediate packaging, outer packaging and package leaflet. |
| Summary of Product Characteristics (SPC) | Contains information on the product as agreed during the course of the assessment. |
| Mock-up | A flat artwork design in full colour, presented so that it provides a full-size replica of both the immediate, outer packaging and package leaflet so that the three dimensional presentation of the label text is clear. |
Templates
You must provide draft QRD text using the appropriate template. For initial national Marketing Authorisations (MAs) validated on or after 29 April 2024, you must use the . An is available which provides supporting guidance on the information requirements listed.
For national MAs granted or validated before 29 April 2024, you may continue to use prior versions of this template, available here:
- For MAs validated before 28 January 2022, the original national product information template (MS Word Document, 58.9 KB)
- For MAs validated after 28 January 2022 but before 29 April 2024, the or national product information template. V.1 was updated on 13 June 2023 to simplify requirements for the outer and immediate labelling
- For national MAs, you should update and implement the product information in line with the v.3 template by 1 April 2029.
For mutually recognised MAs where NI is Concerned Member State (CMS), guidance on QRD templates is available from the EMA website.
See Summary of Product Characteristics and product literature for veterinary medicines for more information on the application process relating to mock-ups in the UK.
Before you create mock-ups
The amount of space you have available on packaging should be considered from the outset and throughout the assessment process. The amount of text included on the QRD must reflect the size of the label.
If you are concerned about fitting the agreed text onto your packaging you must discuss this with the relevant assessor during the assessment phase before the QRD text is agreed.
Designing mock-ups
The following should be taken into consideration during the design of mock-ups.
Font type and size:
- font type should be easy to read and not overly stylised, which can be difficult to read
- choose a font that clearly distinguishes between similar characters such as i, l and 1
- capitals should be avoided but can be used to emphasise words
- Italics should be used for Latin terms when citing correct nomenclature and microorganisms
- font size should be as large as possible and must be clearly legible to the user. Font size should be measured against Times New Roman. If you can’t use the recommended font sizes list below, please include a justification for this when submitting mock-ups
| Type of Packaging | Recommended Font Size | Minimum Font Size |
|---|---|---|
| Small Immediate Pack Sizes | 7 pt | 4.75 pt* |
| Immediate Packaging | 7 pt | 6 pt |
| Outer packaging | 7 pt | 7 pt |
| Package Leaflet | 9 pt | 8 pt |
*only in exceptional circumstances and on a case-by-case basis.
Colour
- colours should have a good contrast between the text and the background
- legibility of information should not be compromised by the colours chosen. Preferably, dark text should be printed on a light background, but the reverse may also be applied in certain circumstances, such as to highlight a particular warning
Design and Layout
- legibility of information should not be compromised by design. Consider whether the layout, font size and legibility has been fully optimised
- where possible line spaces should be kept clear which enhances clarity
- column text formatting is acceptable but the margin between columns should be large enough to separate the text clearly
- if space is limited a vertical line may be used to separate text. Related information should be kept together so that information flows from one column to the next
Headings
- bold type and different colour headings can help information stand out and users navigate the text
- spacing above and below the headings should be consistent throughout the packaging
- avoid using multiple headings as using more than two levels may cause confusion
- use of QRD headings on the immediate and outer packaging is not obligatory, but you must include headings that clearly convey meaning, such as ‘withdrawal period’
Use of Images and Symbols
- you can include clear diagrams and images in addition to wording, provided they are not misleading or cause confusion
- symbols and images can be useful provided the meaning is clear and that the size of the image is legible
Shared labelling with other countries
Shared labelling are medicinal products that are labelled to allow their placing on the market in several countries with the same packaging.
Links to the Product Information Database
If you wish to share a label/leaflet with another country, the following statement must appear on your leaflet to help UK users find more product information:
‘Find more product information by searching the ‘Product Information Database’ on www.gov.uk.’
This statement may be omitted where reference to the Union Product Database is not included.
Joint assessment of labelling with Ireland
Joint assessment refers to the procedure whereby a shared label/leaflet is jointly assessed and agreed by the VMD, on behalf of GB and NI, and the Health Products Regulatory Authority (HPRA) for use on veterinary medicines marketed in their territories.
See Joint labelling for veterinary medicines for use in the UK and Ireland.
Multilingual Labels
Multilingual labels refers to the use of two or more languages for at least one component of the packaging material. The inclusion of multiple language text must ensure that:
- requirements of the VMD are respected
- legibility of the English text is not compromised
- information given is identical in all languages. The VMD will only assess the English text on the label
Exception for Centralised Procedure Labels
For veterinary medicines authorised under the Centralised Procedure in the EU and where a label is shared with GB, the VMD will allow you to omit the name and address of the GB marketing authorisation holder/distributor (if different to the EU marketing authorisation holder information) and the GB Vm number on the accompanying package leaflet, subject to this GB information appearing in a national specific box on the outer packaging (or immediate packaging if no outer packaging).
The country abbreviations GB or UK(GB) and UK(NI) are acceptable for use on all packaging and leaflets. The abbreviation ‘XI’ for Northern Ireland should not be used.
Labelling requirements
If it is not practical to include all the information on the immediate or outer packaging, a package leaflet must be included and the statement ‘Read the package leaflet before use’ should appear on the immediate and outer packaging.
The front and back of the label should include all QRD requirements for an immediate label/outer carton. This ensures that if the front portions of the label are torn off, the rear label will retain the minimum required text. If the immediate label is small, assessors may agree for reduced text to be applied on a case-by-case basis.
It is important that the information on a blister pack remains available to the user up to the point when the last dose is removed. Often, it is not possible to display all the information over each blister pocket, therefore, random displays of information should appear frequently. It is acceptable to apply the batch number and expiry date at the end of the blister.
National specific information should be included in the country specific box on shared labels. This should include any information that is specific to that country only. Any information that applies to both countries should be included in the main text outside this box.
Strength and total content
Sometimes packaging may need to include both quantity per unit volume and total quantity per total volume. Different strengths of the same product should be expressed in the same format.
Unless space is limited, micrograms should be spelt out in full. Trailing zeros should not be used, for example 2.5 mg not 2.50 mg.
Company websites
You can’t refer to company websites. You can include an email address and/or telephone number.
Anthelmintics
In the UK, we have a voluntary labelling scheme for the inclusion of chemical group symbols on sheep anthelmintics. You can also use the scheme for other species.
Through the use of approved symbols, users can easily identify the chemical group which helps efforts to delay the development of anthelmintic resistance. The symbol should in a prominent position on the outer carton, immediate label and package leaflet.
A minimum diameter of 10 mm is suggested for the symbol on the outer carton, which can be scaled up for larger presentations.
No symbol is required for narrow spectrum anthelmintics such as closantel.
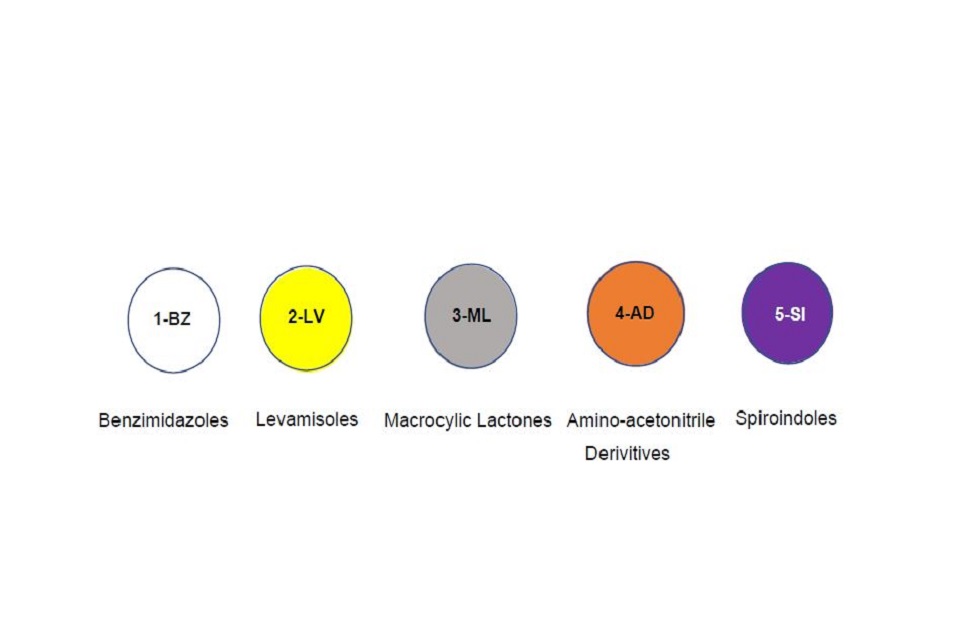
Examples of approved symbols and the diameters which should be used.
QR Codes
A QR code or 2D barcode may be added providing legibility of the required text is not compromised and only accesses:
- information intended for internal manufacturing processing, stock control or anti-counterfeit measures that cannot be accessed by the public
- public information which conforms to the VMD (or HPRA for joint labels) approved product information (Summary of Product Characteristics, label or package leaflet for that product). Links to company websites are considered promotional and cannot be included.
To add a QR code to existing packaging you will need to submit a G.I.15.z Variation Requiring Assessment. The variation must include a detailed account of information to which this code links and any significant changes to this information would require the submission of a further variation.
Once a QR code has been approved the MAH is responsible for ensuring that the information included in the QR code is in line with the approved SPC.
Product ranges
There should be a separate SPC and product literature for each strength and pharmaceutical form of a veterinary medicine.
You may be able to combine package leaflets for products that are different strengths and/or different pharmaceutical forms. If a combined package leaflet is required, this should be raised during the assessment phase when the QRD text is being agreed and before the mock-up review process.
A combined package leaflet will only be agreed if the contents are identical for each product and the combination does not cause confusion for the user.
Mock-ups requirements
This section describes the specific details that must appear on each of the different packaging types.
If space is available, you may be asked to include extra information especially in the case of Provisional and Limited Marketing Authorisations.
Names and addresses of MAH, site of batch release and distributors
| Details to appear | Packaging type |
|---|---|
| Name only or company logo | Outer Packaging, Immediate Packaging |
| Name and address | Package Leaflet, Label if no leaflet |
| Batch release site if different to the MAH | Package Leaflet, Label if no leaflet |
Where several company names and addresses appear, the role of each should be clear. If space is limited the addresses can be shortened; however, it must include the name of the country if outside the UK (or Ireland for joint labels).
A local representative may also be included on the package leaflet and label if no leaflet, but this is not a legal requirement.
You may include the details of a named distributor on your labels instead of, or as well as the MAH details.
Product name followed by its strength and pharmaceutical form
This information must match the SPC/QRD and appear on each packaging type. The whole product name should appear together. Only the invented name is required on small immediate packaging.
Copyright or trademark symbols are allowed.
Name and quantity of the active substance and any excipient
| Details to appear | Packaging type |
|---|---|
| Name and quantity of the active substance and excipients if shown in Section 2 of the SPC | Package Leaflet, Label if no leaflet |
| Name and quantity of the active substance | Immediate Packaging, Outer Packaging |
| Quantity of the active substance | Small Immediate Packaging |
Indications, dosage and adverse reactions
| Details to appear | Packaging type |
|---|---|
| Indications: Non-prescription product | Package Leaflet, Outer Packaging, Label if no leaflet |
| Indications: Prescription products | Package Leaflet, Label if no leaflet |
| Contraindications | Package Leaflet, Label if no leaflet |
| Adverse events | Package Leaflet, Label if no leaflet |
| Target Species: For small immediate packaging it is strongly recommended to include a pictogram of the target species | Package Leaflet, Outer Packaging, Immediate Packaging, Label if no leaflet |
| Dosage | Package Leaflet, Label if no leaflet |
Route of administration, dates and warnings
The method or route of administration should be written as per the SPC. Standard abbreviations are acceptable on small immediate packaging or on the outer packaging provided that full terminology is used on the package leaflet. Non-standard routes should be written out in full.
| Details to appear | Packaging / Leaflet type |
|---|---|
| Route of administration | Outer Packaging, Immediate Packaging, Package Leaflet, Label if no leaflet |
| Advice on correct administration | Package Leaflet, Label if no leaflet |
| Withdrawal period: For food producing species the withdrawal period, as per the agreed QRD should be shown even if it is zero hours/days | Package Leaflet, Outer Packaging, Immediate Packaging, Label if no leaflet |
| Special storage instructions | Package Leaflet, Outer Packaging, Immediate Packaging, Label if no leaflet |
| Special warnings: Warnings as per the following sections of the SPC – 3.4, 3.5, 3.7, 3.8, 3.10, 3.11 and 5.1 | Package Leaflet, Label if no leaflet |
| Disposal advice: As written in Section 5.5 of the SPC unless otherwise agreed in the QRD. If agreed during assessment, additional national disposal and environmental warnings may also need to be included on the packaging | Package Leaflet, Label if no leaflet |
| The statement: ‘Find more product information by searching for the ‘Product Information Database’ on www.gov.uk.’ This statement is only required where reference to the Union Product Database is also included. | Package Leaflet, Label if no leaflet |
| Other information: Further information required in the MA | Package Leaflet, Label if no leaflet |
| Batch Number, preceded by the word “Lot” | Outer Packaging, Immediate Packaging, Small Immediate Packaging, Label if no leaflet |
| Expiry Date: The expiry date should be written clearly to avoid confusion. Dates may be printed, embossed or engraved into the packaging. If this is overprinted onto the final printed mock-up this should be clarified to the competent authority | Outer Packaging, Immediate Packaging, Small Immediate Packaging, Label if no leaflet |
| The in-use shelf life (if appropriate) | Outer Packaging, Immediate Packaging, Small Immediate Packaging, Label if no leaflet |
Intended use
| Details to appear | Packaging / Leaflet type |
|---|---|
| The words ‘For animal treatment only’ | Outer Packaging, Label if no leaflet |
| The words “Keep out of the sight and reach of children” | Outer Packaging, Package Leaflet, Label if no leaflet |
| The words “Read the package leaflet before use” | Outer Packaging, Immediate Packaging |
Marketing Authorisation details
| Details to appear | Packaging / Leaflet type |
|---|---|
| Package size | Outer Packaging, Label if no leaflet |
| Classification of the veterinary medicine, including the distribution category. The distribution category should appear in a box. | Package Leaflet, Label if no leaflet |
| The marketing authorisation (MA) number. The package leaflet and label if no leaflet should also contain a list of authorised pack sizes. | Package Leaflet, Outer Packaging, Label if no leaflet |
For shared labels, where possible and when space allows, country specific information should appear in a box like this on mock-ups:
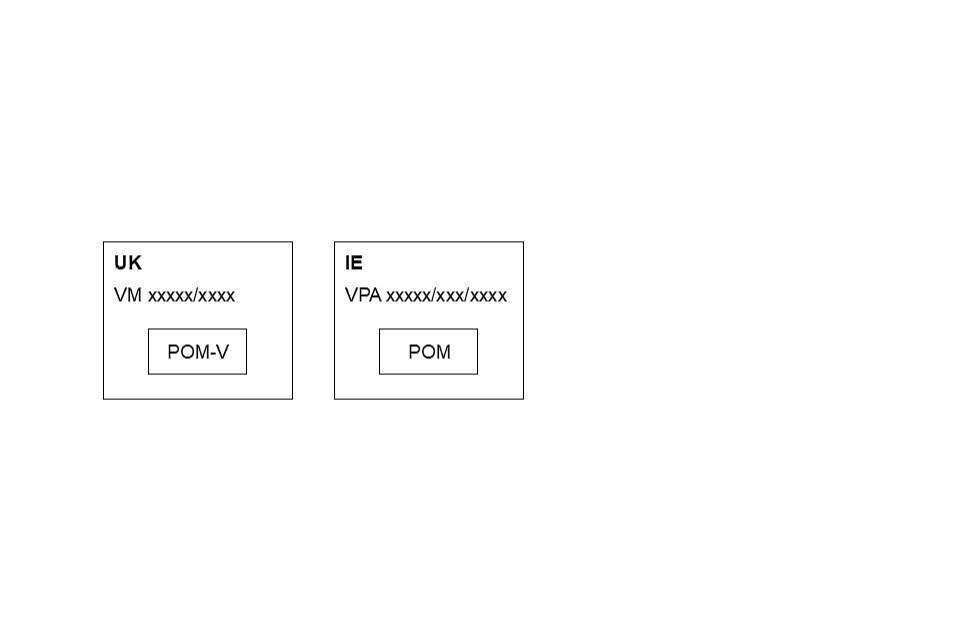
Diluent label
The minimum amount of information which should appear on a diluent label:
- Name of diluent: The ‘trade’ name with a brief description or a more descriptive way of naming (Solvent /diluent for type of vaccine it can be used with or properties of the diluent)
- Target species
- Route(s) of administration: The statement ‘Read the package leaflet before use’
- Storage conditions: For example: Store below 25ºC
- Batch Number
- Expiry Date
- Name or company logo of the Marketing Authorisation Holder
Veterinary Homeopathic Registrations
The labels of a registered Veterinary Homeopathic product must have the following:
- Clear mention of the words “homeopathic remedy without approved therapeutic indications for veterinary use”
- Scientific names of the stock(s) followed by degree of dilution using symbols of pharmacopoeia. If product is composed or more than one stock, the packaging may need to mention an invented name in addition to the scientific names of the stocks
- Name or company name and the permanent address or registered place of business of the registration holder and of the manufacturer
- Method and, if necessary, route of administration. The route of administration should be included on small containers, up to 50 ml.
- Expiry date
- Pharmaceutical form
- Contents of the pack
- Special storage precautions
- Withdrawal period, where applicable, or a statement if the product is contraindicated for animals intended for human consumption
- Target species
- A special warning for the product, if necessary
- Manufacturers batch number
- UK only: a registered product will have registration number preceded by the symbol Vh on the labels
- IE only: A registered product will have a registered number in the following format: HoVR 01/001
Controlled Drugs
Products containing controlled drugs listed in Schedule 2 or 3 of the Misuse of Drugs Regulations 2001 must be clearly identified with “CD” either in a triangle (preferably) or in a box with the relevant schedule below.
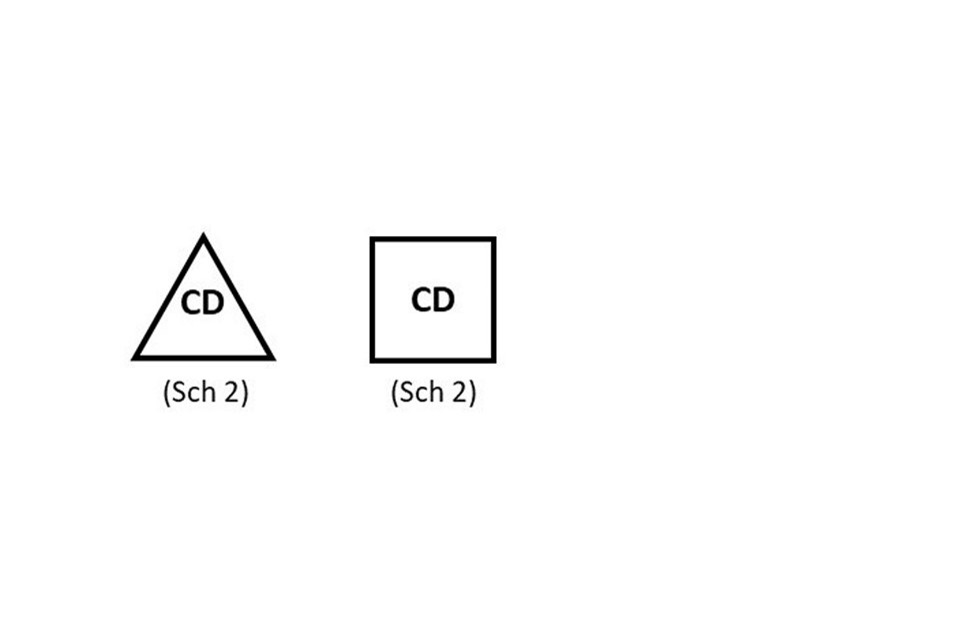
“CD” should appear either in a triangle (preferably) or in a box with the relevant schedule below.
The CD symbol should be included on labels (where space permits), outer packaging and on the package leaflet. This does not need to be included on blister packs or small immediate labels.
Dedicated Dispensing Containers
If submitted to the VMD with the authorised packaging, they are considered to form part of the authorised packaging and will be subject to the same level of mock-up assessment.
Mock-ups assessment
Mock-ups supplied in support of an application to the VMD must be in accordance with the Product Literature Standard and presented as a flat artwork design in full colour. We will assess all mock-ups submitted in support of a national GB and/or NI application. This includes assessment of mock-ups for GB national applications submitted in parallel to an EU centralised procedure.
Mock-ups are assessed against the agreed QRD text and SPC, the Product Literature Standard and any other information provided by or discussed with you.
Mock-ups submitted should not deviate from the agreed QRD text and/or SPC. However, in exceptional cases, you may be allowed to deviate but you must contact us to discuss this before submitting your mock-ups. Not doing so will delay the approval of your mock-ups and subsequent issue of the application.
The following are not assessed and should not be submitted with applications:
- shipping packs
- datasheets, including Material Safety Data Sheets (MSDSs)
- packaging for wholesalers that do not include any labels
- display packaging
- promotional material
- dispensing envelopes are not required. However, if submitted to the VMD with the authorised packaging, they are considered to form part of the authorised packaging and will be subject to assessment
No mock-ups
If you do not want to submit mock-ups (for certain pack sizes), or mock-ups are not submitted within the deadlines set, your application will be issued with a condition that mock-ups will need to be submitted for assessment under cover of a national G.I.15z variation requiring assessment (VRA), prior to marketing.
Mock-up changes that require a variation
Any changes not already covered by a specific variation classification that may affect the legibility of the mock-up must be approved by way of a national G.I.15z variation requiring assessment (VRA), for example:
- new corporate design of packaging
- new container type / size
- changes to the layout of the package
- introduction of multilingual packs for an already approved authorised product
- introduction of mock-ups for an authorised pack size
You should only submit the mock-ups for the packaging/labelling that is affected.
Notifications
Minor mock-up changes that do not affect the font size, layout or legibility do not require a variation, for example a change to the barcode, logo (assuming same size), change of IE national specific text.
If you are in doubt about whether a variation is needed, please contact notification@vmd.gov.uk.
We will then confirm if this is acceptable or if a variation is required and make a note of the changes in our records. This does not mean the VMD has assessed or approved the described change(s) only that they can be made without the need for a variation. You are not required to submit revised mock-ups to the VMD but they can help to clarify the change.
Assessment timescales
Mock-ups will be assessed within 20 days of receipt of correct versions.
If only minor amendments to existing approved mock-ups are needed we will annotate your existing approved mock-ups, otherwise new mock-ups will be requested. You will be notified of this within 4 days of the procedure ending.
If the mock-ups contain errors, or are not suitable, you will receive a comments document listing any proposed changes. You should update the mock-ups to incorporate the proposed changes and return revised versions as soon as possible.
You will only receive 1 comments document. If the revised mock-ups are still incorrect a condition will be applied to the authorisation to introduce corrected mock-ups under the cover of a suitable national variation prior to marketing.
Updates to this page
Last updated 29 April 2024 + show all updates
-
Updated to reflect changes to the VMR.
-
Updated VMD and HPRA guidance and templates on acceptable text for joint-labelled veterinary medicines in the UK and Ireland.
-
Update to the Templates section and reference for Mock-ups that require a variation.
-
Added section 'Multicountry Labels for Marketing Authorisations Granted under the Centralised Procedure.'
-
First published.